

How’s the Air Up There?
For more than 40 years, scientists in a tiny Central Otago pitstop have been studying the ozone layer and the carcinogenic UV rays from the sun. Their critically important work has literally helped save our skins.
By George Driver
It looks like a cult. At 9.20am on a frosty September morning in a paddock surrounded by farmland and snow-capped peaks in Central Otago, two people are dressed head-to-toe in orange coats and matching face masks. Together they patiently fill an enormous balloon with hydrogen gas. Once it is inflated to the size of a Mini Cooper, the pair attach a small cardboard box. Inside is a device that will take small samples of the atmosphere, producing an electric charge every time an ozone molecule is sucked through a small tube. A radio transmitter on the side of the box sends that signal back to Earth where it is logged on a computer at the Lauder Atmospheric Research Station, an outpost of the National Institute of Water and Atmospheric Research (NIWA).
“This is the premier measurement for ozone profiles,” explains Richard Querel, who manages the Lauder NIWA station. “It’s tasting the ozone layer where it actually is.”
At 9.36am, the rubber balloon is released, rising at five metres a second. In less than an hour it enters the stratosphere, “tasting” the diffuse ozone layer as it rises, before it expands in the rarefied air 33km up and explodes, allowing its payload to parachute down. Most likely its component parts will end up in the Pacific Ocean, never to be seen again . Querel says most of the components are biodegradable, except for the metal electronics.
It’s a ritual which has been carried out in Lauder every Tuesday morning — weather permitting — for the last 36 years. It is just one of a dozen or so measurements scientists there regularly make of the ozone layer and the gases which threaten it, making Lauder one of the most important atmospheric research stations on the planet.”
When these measurements began in 1986, the stakes were almost existential. The ozone hole above Antarctica had just been discovered, shocking scientists and the public alike. The shield of the ozone layer, on which life on this planet depends, was more fragile than anyone had imagined. New Zealand, with the population area closest to the “hole”, was on the front lines. No one was quite sure what would happen next. Scientists at Lauder helped find out.
A FRAGILE SHIELD
Ozone is strange. It’s a pollutant when it occurs at ground level, but when it is in the stratosphere it creates a shield which stops most of the sun’s DNA-damaging UV rays.
It is composed of three oxygen atoms, O3, one more than normal oxygen, O2. At ground level, ozone can be formed by sunlight breaking apart molecules emitted from burning fossil fuels and is a part of smog, causing lung damage and stunting plant growth. But about 90 per cent of Earth’s ozone is in the stratosphere, a layer between about 10km and 50km above the Earth’s surface. Here it is formed by a natural process where powerful ultraviolet sunlight splits apart O2 molecules, which recombine with other oxygen molecules to form O3.
There’s not much of it up there. If all of the ozone in the atmosphere was squished down to ground level it would form a layer just 3mm thick. At its highest concentration, at an altitude of about 25km, ozone makes up just 0.0015 per cent of the atmosphere. But these diffuse molecules absorb approximately 90 per cent of the invisible radiation called UVB that causes sunburn and skin cancer (There are two other forms of UV: UVA rays, which aren’t filtered by ozone and are linked to skin ageing rather than cancer; and UVC rays which are entirely blocked by ozone. The difference between the UVB and UVA levels is one way to measure the thickness of the ozone layer.)
Until recently, the ozone layer was of passing interest to scientists. Andrew Matthews worked at Lauder for almost two decades. When he began his PhD at the University of Canterbury in the late 1960s, looking at methods of measuring the ozone layer, he says it was mainly studied to help predict weather systems. “The ozone layer was thought to be constant and inert to chemical reactions,” he recalls.” He would soon find himself amid one of the greatest scientific debates of the 20th century.”
In the early 1970s, Dutch scientist Paul Crutzen theorised that a boost in nitrogen oxides in the stratosphere — either from fertiliser or a future fleet of supersonic aircraft — could cause chemical reactions that destroy ozone. The US, UK, French and Soviet governments were spending hundreds of millions of dollars developing supersonic passenger planes, which would fly up in the stratosphere where there’s less air resistance, but also where the protective ozone layer resides. The ozone layer started making headlines around the world, the New York Times writing in 1972 that supersonic planes “could endanger Earth”.
Scientists then started asking what other gases might impact ozone. A couple turned their attention to synthetic molecules called chlorofluorocarbons (CFCs), found in aerosol cans and refrigerators around the world.
CFCs were actually invented to reduce harmful pollution. They were developed by Thomas Midgley Junior, who was dubbed by New Scientist a “one-man environmental disaster”. While working for General Motors in the 1920s, Midgley first developed leaded fuel, an invention that caused elevated levels of poisonous lead worldwide and has been associated with health issues affecting millions of people. Later in the decade, he turned his attention to developing a new refrigerant with arguably more disastrous consequences.”
At the time, refrigerators relied on toxic gases, for which synthetic chlorofluorocarbons provided a much safer alternative, being non-toxic, non-flammable and unreactive. As Bill Bryson writes in A Short History of Nearly Everything, “seldom has an industrial product been more swiftly or unfortunately embraced”. CFCs began to be used widely in refrigerators, air conditioners and as a propellant in aerosol cans containing everything from whipped cream to deodorant. Production skyrocketed. Incidentally, Midgley was later killed by one of his own inventions. In 1944, he was strangled by a mechanism he designed to help him out of bed.
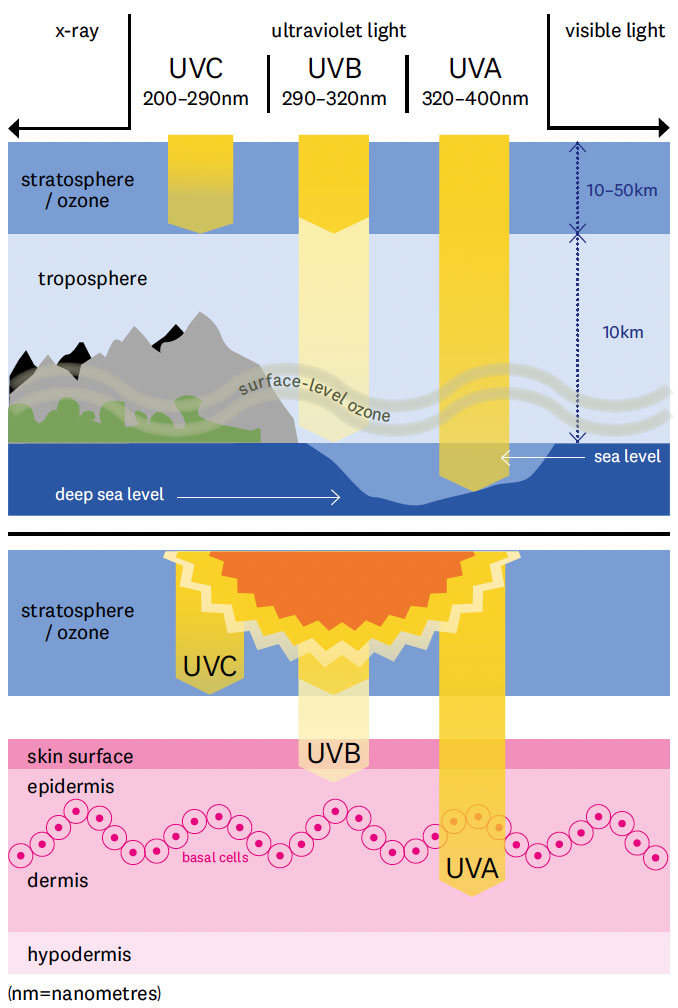
Diagram: Imogen Greenfield
Ultraviolet (UV) electromagnetic radiation from the Sun occurs at di!erent wavelengths which are blocked by the ozone layer at di!erent rates. UVA rays have the least amount of energy and aren’t blocked by the ozone layer at all, but are mainly linked to skin ageing rather than skin cancer. About 90 per cent of UVB rays are filtered by ozone, but it is higher intensity and is thought to cause most skin cancers. UVC rays have the highest energy but are entirely blocked by ozone.
It wasn’t until the early 1970s that it was realised that the chemical stability of CFCs could be a problem. They take decades to degrade and there’s no natural “sink” to break them down, Matthews explains. Because of this, they became a useful marker showing the diffusion of man-made chemicals. Irish scientist James Lovelock invented a device — the electron capture detector — that could detect chemicals at very low concentrations in the atmosphere and in the early 1970s he started looking for CFCs. He found them everywhere he looked. CFCs were even in Antarctica, showing that man-made chemicals had found their way right around the globe.
On hearing Lovelock’s research, Californian chemistry professor F Sherwood Rowland began to investigate what would happen if these supposedly benign CFCs made it up into the stratosphere. It wasn’t good. In a short paper published in Nature in 1974, Rowland and his post-doctoral fellow Mario Molina theorised that CFC molecules could be pried apart by UV light in the stratosphere, freeing chlorine atoms that could destroy ozone at an astonishing rate. It’s since been calculated that just one chlorine atom can destroy over 100,000 ozone molecules.”
If rates of CFC production plateaued, Rowland found this could lead to a six per cent reduction in the ozone layer, increasing skin cancer rates by roughly 12 per cent. But CFC production was rising exponentially.
Andrew Matthews was on his own post-doctoral fellowship at a Max Planck Institute in Germany at the time and went to a lecture by Rowland in Cologne. He recalls it being something of a turning point for science.
“There were all of these yellow flyers throughout the auditorium that said ‘The talk you are about to hear is a whole lot of bunkum — it’s not substantiated, but a figment of a scientist’s imagination.’ Science at that stage wasn’t really political and I’ve never forgotten it. But that was the chemical industry, girding their loins.”
The idea that CFCs could destroy the ozone layer was still just a theory. But it set in motion international research that would lead to the most successful environmental agreement in history, preventing millions of skin cancers.

Lauder station manager Richard Querel is helping to develop methods of measuring farm methane emissions in real time, rather than calculations based on models. Photo: courtesy of NIWA

Now retired, Lauder scientist Richard McKenzie, above, helped develop an app, UVNZ, which shows UV levels at your location in real time. Photo: courtesy of NIWA
RESEARCH AT THE END OF THE EARTH
Lauder’s a blink-and-you-miss-it kind of place. It was a stop on the Otago Central Railway between Dunedin and Cromwell — a dozen houses, a pub and a school — in between a place called Becks and a place called Omakau. But in the 1980s the trains stopped coming and the school closed. The railway line was finally ripped up in 1991. The pub limped on.”
But there’s something that makes this part of the world exceptional, attracting artists, poets, cyclists and some of the world’s top atmospheric scientists. “It’s the cleanest air on Earth,” Richard Querel says. The air from the west is “washed” by rain, which falls in torrents to the west of the Southern Alps. The fog and cloud from the east coast is kept at bay by waves of ranges — the Raggedy, Rough Ridge, the Rock and Pillar and the Hawkduns. In the middle is a basin that is almost, but not quite technically, a desert. It’s a kind of Goldilocks zone for air quality — not dry enough to create lots of dust, not moist enough for pollen or haze.
Consequently, there’s a clarity to the light in Central Otago that is astounding. As anyone who has biked the Otago Central Rail Trail or viewed a Grahame Sydney painting knows, the surrounding landscape is rendered in vivid detail. Readings on the light spectrometers and other instruments used to study the atmosphere are likewise of a quality found almost nowhere else.
Lauder also fills a hole in atmospheric research, Querel explains. There are very few places in the mid- latitudes of the southern hemisphere with instruments trained on the stratosphere, and institutions from Washington to Amsterdam have spent millions ensuring Lauder is well equipped to provide high- quality long-term data.
The research station was established in 1961 following the International Geophysical Year in 1957, a project to renew international cooperation on Earth sciences, which had been hampered by the Cold War. The site was initially set up to study the Aurora Australis, or the Southern Lights, which could disrupt radio communication and navigation around the world. Lauder’s one of the best places to research the Aurora, without actually going to Antarctica.
Richard McKenzie, who got a job at Lauder in 1979, recalls that by then it was already a backwater. Half of the eight staff lived onsite — including McKenzie — in what he describes as a “motley collection of five houses spread along a couple of hundred metres of road”. The laboratory buildings were mostly old Ministry of Works huts, but with a new office block, set amidst farmland. An old military truck had been converted into an observatory — it was later sold for $1. He says the local farming community nicknamed the scientists “the stargazers” and didn’t really know what the research station was for.
Satellites had made auroral research less necessary and Lauder’s importance was on the decline. But visiting researchers had started making the first southern hemisphere measurements of nitrogen dioxide in the atmosphere, attempting to understand whether supersonic planes could destroy ozone — the Concorde had just started plying the Atlantic three years earlier. McKenzie joined the research, measuring nitrogen dioxide above Lauder. Matthews followed three years later, joined the research and went on to become station manager. What they discovered helped scientists understand that CFCs were the true cause of ozone depletion, helping to convince the public and politicians to act.

Photo: George Driver
The NIWA research station at Lauder. The site also includes this dome with a satellite dish, instruments to download information from passing satellites and a telescope used to record what happens when black holes collide.
A HOLE IN THE SKY
When it was first theorised that CFCs were damaging the ozone layer, the response was surprisingly rapid. By 1978, the US, Canada, Sweden and Norway banned CFCs in most aerosols (butane provided an easy alternative for aerosols but there were no ready alternatives for refrigerants). But because ozone is subject to natural variations, there wasn’t yet any hard evidence that the ozone layer was being depleted. The industry played up this doubt and production of CFCs continued to climb.
“We’ve since seen the same with the greenhouse- gas industry,” Matthews says. “In retrospect, they were playing for time until they could develop an alternative.” However, by the 1980s there was a growing international consensus that CFCs were likely to cause ozone depletion over the coming decades and should be phased out. In March 1985, countries gathered in Vienna to sign the first international agreement on CFCs, dubbed the Vienna Convention. It was still relatively weak, calling on countries to take action “should it be found that these activities [CFCs] have or are likely to have adverse effects,” putting the burden of proof back on scientists.
Two months later, everything changed. British scientist Joe Farman had been measuring the ozone layer above Antarctica and found ozone levels had started plummeting by up to a third in spring. He published a paper in Nature in May 1985 claiming CFCs were to blame. The lack of sunlight during the long polar winter in Antarctica and the swirling westerly winds, called the polar vortex, causes temperatures in the stratosphere to plummet below -80C, freezing polar stratospheric clouds. The ice crystals in these clouds provide a surface for chemical reactions with CFCs when the first UV rays hit in spring, supercharging the destruction of ozone. When the polar vortex weakens in November the ozone hole breaks up and ozone levels return to normal, but for a few months each year the amount of ozone can drop by up to a third.
By August 1985, NASA satellite data was able to show ozone levels had been declining over the entire continent each spring since the early 1980s. The space agency had launched a satellite to monitor ozone in 1978, but Matthews says, embarrassingly, the algorithm used to analyse the data had been designed to discard low readings as errors. Were it not for this, the hole may have been detected earlier.
Ozone depletion was no longer a theory. It was happening and at a much faster rate than anyone thought possible. Matthews says it fundamentally changed how people viewed our impact on the environment. “For the first time, humankind was shown to be able to affect the atmosphere on a global scale.”
Richard McKenzie was writing his PhD in Oxford at the time and attended a lecture by Farman just before the landmark paper was published. In his book Saving Our Skins, he writes that at this moment “our friends and family were on the front line” and Lauder was “right at the centre of the action”. “It galvanised the world into action,” he recalls.
Despite the discovery of the ozone hole, debate continued on whether CFCs were to blame or whether it was caused by natural phenomena linked to increased solar activity. Some even questioned whether the “hole” mattered at all.”
“One Texas senator at the time said ‘Why should we sacrifice our industries when this is only affecting a few blimmin’ penguins,’” Matthews says.
Research at Lauder played an important role, showing that variations in ozone caused by sunspots were not a major factor causing the ozone hole and therefore CFCs were likely to blame. Further proof came in 1987, when a NASA plane flew through the stratosphere over Antarctica as the ozone hole formed and confirmed CFCs were abundant.”
A paper by Matthews meanwhile showed the ozone hole was leading to low ozone air passing over New Zealand in late spring, after the ozone hole breaks up, potentially leading to elevated UV levels and higher cancer rates.”
The number of instruments at Lauder measuring ozone and the “trace gases” that cause its destruction started to grow as scientists around the world raced to understand what was happening above Antarctica. Lauder became a common diversion for experts on the trip between the US and Antarctica, which always stopped in Christchurch, a five-hour drive from the Lauder station. These connections have ensured Lauder now has more methods of measuring the ozone layer than anywhere else.
“We’re a supersite for ozone research,” Querel says. “Lauder is a bit of a Rosetta Stone for the different techniques. Because we are looking for changes of just a few per cent each decade, we need to ensure we understand the differences between different measuring techniques. Lauder allows us to do that.”
After the ozone hole was discovered, political action was again relatively rapid. By 1987, the Montreal Protocol was signed with countries agreeing to reduce CFCs by 50 per cent by 1999. Three years later, as the ozone hole continued to expand, developed countries agreed to phase out CFCs entirely by 1996 and global production largely ended by 2000.
CFCs released decades ago continue to make their way up into the stratosphere, but by the mid 1990s their levels peaked and have gradually declined since. The size of the ozone hole peaked in 2006, when it covered 27 million square kilometres. It’s stabilised and slightly declined since — although the last couple of years it’s been larger due to colder temperatures in the stratosphere. Interestingly, while climate change leads to warmer temperatures in the lower reaches of the atmosphere, McKenzie says the greenhouse effect actually cools the stratosphere as greenhouse gases prevent warm air from ascending. But as the amount of CFCs continues to shrink, the ozone hole is expected to disappear entirely by about 2080.
While people often contrast the success of the Montreal Protocol with the slow progress of phasing out greenhouse gases, McKenzie says the key difference was the CFC industry was able to develop profitable alternatives. “The people who caused the problem could make money by fixing it — that’s the difference between climate change and ozone depletion.” Matthews also points out that while CFCs were a billion-dollar industry, their importance was relatively minor compared to fossil fuels.”
“It was relatively easy for people to use roll-on deodorant rather than a spray compared with asking people to reduce their use of fossil fuels.”
CARCINOGENIC RAYS
With the causes of ozone depletion finally beyond debate and CFC levels set to decline, research at Lauder turned to studying how this was impacting UV radiation in New Zealand.
Despite more than a decade of international research on ozone depletion, McKenzie says in the late 1980s there were few ways of measuring changes in UV and little relevant research had been done. In New Zealand, UV measurements had only been undertaken in Invercargill and these weren’t particularly useful due to frequent cloud cover. But Lauder’s clean air and clear skies made it an ideal UV research site and it became one of the first in the world to have accurate long-term data.
McKenzie and colleague Paul Johnston developed a purpose-built UV spectrometer in the late 1980s which still forms the basis for NIWA’s UV measurements and is used by scientists around the world. These recordings helped show how ozone depletion was leading to elevated UV levels here. In 1999, McKenzie and a NIWA colleague published a paper showing UV levels had increased by five to 10 per cent over the decade. He says it was the first paper to definitively establish the long- term impact of ozone depletion on UV levels.
But over the last two decades the situation has started to turn around, albeit slowly. In 2019, McKenzie and others published a paper in Nature showing if it weren’t for the Montreal Protocol, UV levels would have increased by 20 per cent in New Zealand and would be on track to quadruple by the end of the century. That would mean the average burn time would be about four minutes.
“You would get sunburned crossing the road,” McKenzie says. “But because of the success of the Montreal Protocol, the increase in UV has been small. We averted an absolute disaster.”
MELANOMA MYTHS
New Zealand is still exposed to elevated levels of UV, but it’s not as bad as you might think and, contrary to popular belief, New Zealand is no more affected by ozone depletion than anywhere else.
While New Zealand has the world’s highest rates of melanoma, jockeying for the title with Australia, McKenzie says the thinning ozone layer has been a minor factor. “Compared with the normal day-to-day variations, it’s a small impact,” McKenzie says.
He says the mid-latitudes of the northern hemisphere have experienced a similar level of ozone depletion to New Zealand and the ozone hole over Antarctica has little impact on UV levels here. At its greatest extent, the hole is still more than 1000km to the south of the country, and when it forms each spring, New Zealand actually has among the highest ozone levels on Earth. This is because the polar vortex which allows the ozone hole to form has the effect of concentrating ozone over New Zealand. When the ozone hole breaks up in November, some ozone depleted air can pass over New Zealand, but the effects are small and difficult to detect as it is similar to natural variation, McKenzie says.
Interestingly, when the ozone hole is present over Antarctica, UV levels there are extreme for its latitude, but they are still not particularly high by global standards because the angle of the sun is very low. At the South Pole, UV levels are typically below 1.5 on the UV Index (UVI) in spring and about UVI 3 at Arrival Heights, near Scott Base. That’s about the UV levels experienced in Auckland in mid winter. The highest UV readings in Antarctica are still in mid summer, when ozone levels have recovered but the sun is at a higher angle.
While New Zealand doesn’t experience high UV levels because of ozone depletion, our UV levels are naturally about 40 per cent higher than the same latitudes in North America and Europe.
“These changes [due to ozone depletion] pale in comparison to the huge differences that already existed before the ozone hole and will continue to exist in the future,” McKenzie says.
About half of the difference is because the air is much cleaner in New Zealand. Pollution and dust in the northern hemisphere blocks more UV. Secondly, because of Earth’s elliptical orbit around the Sun, the southern hemisphere is about three per cent (five million kilometres) closer to the Sun in mid-summer (January 4) compared with the northern hemisphere. This makes our UV levels about seven per cent higher. This date changes slowly — in about 10,500 years the northern hemisphere will be closer to the Sun in July — and is a factor in the formation of ice ages. You’d expect the current closer proximity to the Sun to mean the southern hemisphere has a warmer summer, but because 81 per cent of it is temperature-moderating ocean, Earth is actually about 2.3°C colder overall when the south is closest to the Sun.
The third reason New Zealand has higher UV levels is due to the southern hemisphere having naturally lower levels of ozone. Most ozone is produced in the stratosphere over the tropics, where the Sun’s rays are strongest, and differences in wind patterns mean less ozone is transported to southern latitudes.
It’s important to note that while New Zealand has much higher UV levels than Europe and North America, they aren’t particularly high on a global scale. The highest UV levels are in the tropics, where the Sun’s rays hit Earth more directly, and in high altitude areas, like the Tibetan Plateau, where there is less atmosphere to soak up UVB. The highest UV levels in the world are believed to be in Cuzco, 3400m up in the Peruvian Andes where peak UVI can reach 25 — nearly twice that recorded in New Zealand.
Because the angle of the Sun has a large impact on UV levels, Auckland also has much higher UV than Invercargill. Auckland’s mean midday peak UVI in summer is around 10 while in Invercargill it’s around seven. For the same reason, UV levels are much higher in Brisbane and Darwin, where the UVI peaks at 12 and 13 respectively, while in London the mean peak UVI in mid-summer is five.
McKenzie says the most important reason for our higher skin-cancer rates isn’t necessarily our UV levels, but because of our skin types and outdoor lifestyle. Most Pākehā descend from the British Isles, which is at a much higher latitude than New Zealand and consequently has much lower UVI. If Britain was in the southern hemisphere, its northernmost point would still be about 400km south of Invercargill. The antipode (opposite point on the globe) of Auckland is just north of Gibraltar. So in New Zealand, people of northern European descent are getting Moroccan levels of UV, but with Scottish skin types. This is also why Māori and Pasifika melanoma rates are about five times lower than for Pākehā.
While we are relatively close to the equator compared to Britain, New Zealand is also relatively cold because it’s surrounded by a cold ocean. So when it’s 18°C in Invercargill — its average high in January — it’s still getting higher UV radiation than the south of France.
McKenzie says this cooler temperature also encourages “sun-seeking behaviour”. In other parts of the world it’s just too hot to be in the sun when UV levels are highest. This is one theory explaining why our skin cancer rates are slightly higher than Australia’s, even though Australia has much higher UV levels.”
The Montreal Protocol is now hailed as the most successful environmental agreement in history. Rowland, Molina and Crutzen were awarded the Nobel Prize in 1995 for their work discovering that CFCs depleted the ozone layer.”
At Lauder, however, there’s been a price to success. Interest in ozone research has waned and the number of staff there has dipped to nine scientists and technicians, from a peak of 21 in the late 1990s.
Lauder’s research is increasingly turning to climate change, with scientists there developing ways of measuring methane emissions down to the farm level. The staff now mostly live in Alexandra, 35km away, and commute together in a NIWA van — although Querel is living onsite while his house is renovated. The lab is still a series of sheds and a long single-storey 1970s brick office block. Andrew Matthews went on to be a manager at NIWA and served two terms as New Zealand’s natural science commissioner to UNESCO and is now retired in Wellington. McKenzie took voluntary redundancy in 2012, but has continued to spend a day or two at Lauder as an emeritus research scientist and is heavily involved in the international bodies assessing the state of the ozone layer.
He once said he could only happily retire once the ozone-depletion problem was under control. Now he continues his research for the joy of it, knowing the work he was involved with at Lauder helped save our skins.

Photo: Rebekah Parsons-King, NIWA
NIWA scientist Penny Smale operates Lauder’s LiDAR (light detection and ranging) instrument, which fires a series of lasers into the sky to measure the ozone layer.
George Driver is North & South’s South Island correspondent. This role is made possible by support from NZ On Air’s Public Interest Journalism.

This story appeared in the December 2022 issue of North & South.
